DOI: http://dx.doi.org/10.20986/revesppod.2024.1687/2024
REVIEW
Percutaneous electrical stimulation as a pain treatment caused by the most common compressive nerve syndromes in the foot and ankle. Literature review
Estimulación eléctrica percutánea como tratamiento del dolor ocasionado por los síndromes compresivos nerviosos más frecuentes en pie y tobillo. Revisión bibliográfica
Laura Regife Fernández1
Marta Moreno Fresco1
Ramón Mahillo Durán1
1Departamento de Podología. Facultad de Enfermería, Fisioterapia y Podología. Universidad de Sevilla, España
Abstract
Chronic pain triggers emotional, physical, economic, and social consequences for patients, thus causing one of the most costly health problems for society. This unpleasant experience manifests in nerve entrapment syndromes in multiple ways. Therefore, in the face of failed conservative therapies, the introduction of new treatments such as percutaneous electrical stimulation reflects promising results in reducing painful symptoms. Thus, the main objective of this literature review is to assess the effectiveness of the percutaneous electrical stimulation technique in the treatment of neuropathic/chronic pain caused by the most frequent nerve entrapment syndromes in the foot and ankle.
A search has been conducted on various scientific databases during the months of February and March 2023. After applying inclusion and exclusion criteria, a total of 30 articles were selected and analyzed. Of these, 18 articles meeting the search criteria were identified and, consequently, included in the discussion.
In conclusion, it is observed that the technique demonstrates its short-term effectiveness in reducing musculoskeletal, neuropathic, or postoperative pain, as well as medication intake. We have encountered difficulties in demonstrating its effectiveness in nerve entrapment syndromes in the foot and ankle, although it has shown efficacy for those occurring in other parts of the body. Established authors employ varied intervention protocols, with the majority alternating high and low frequencies within a 20-30 minute interval.
Keywords: Nerve entrapment, pain, neuromodulation, PENS, foot, ankle
Resumen
El dolor crónico desencadena consecuencias emocionales, físicas, económicas y sociales para el paciente, ocasionando así uno de los problemas de salud más costosos para la sociedad. Esta experiencia desagradable se manifiesta en los síndromes de atrapamiento nervioso de múltiples maneras. Es por ello que, ante terapias conservadoras fallidas, la introducción de nuevos tratamientos como la estimulación eléctrica percutánea reflejan mostrar buenos resultados en cuanto a la disminución de la clínica dolorosa. De esta manera, el objetivo principal de esta revisión bibliográfica fue conocer la efectividad de la técnica de estimulación eléctrica percutánea en el tratamiento del dolor neuropático/crónico, ocasionado por los síndromes de atrapamiento nervioso más frecuentes en el pie y tobillo.
Se ha realizado una búsqueda en diversas bases de datos científicas durante los meses de febrero y marzo de 2023. Tras aplicar criterios de inclusión y exclusión, se seleccionaron y analizaron un total de 30 artículos. De estos, 18 cumplieron con los criterios de búsqueda y se incluyeron en la discusión.
Como conclusiones se obtiene que la técnica demuestra su efectividad a corto plazo en cuanto a la reducción del dolor musculoesquelético, neuropático o postoperatorio, así como en la ingesta de medicamentos. Hemos tenido dificultades para evidenciar su efectividad en los síndromes de atrapamiento nervioso en el pie y tobillo, aunque sí para los ocasionados en otras partes del cuerpo. Los autores contrastados utilizan diferentes protocolos de actuación. Si bien la mayoría alternan altas y bajas frecuencias en un intervalo de 20-30 minutos.
Palabras clave: Atrapamiento nervioso, dolor, neuromodulación, PENS, pie, tobillo
Correspondence: Laura Regife Fernández
lauraregife@gmail.com
Received: 13-01-2024
Accepted: 09-03-2024
Introduction
Pain is a subjective and complex experience defined by the International Association for the Study of Pain (IASP) as “an unpleasant sensory or emotional experience associated with actual or potential tissue damage, or described in terms of such damage” (1). When it persists and becomes chronic, the pathophysiological effects and behavioral responses it causes differ from those associated with acute pain. Data shows an incidence of 25.7 % (1.5 billion people) overall(2), between 19 % and 31 % in Europe, and 30 % in Spain(3).
Neuropathic pain is chronic pain caused by a disease or injury of the somatosensory nervous system, leading to the manifestation of signs and symptoms specific to hyperexcitability. Among chronic pain types, it is one with a higher prevalence, ranging from 6.9 % up to 10 %(1).
Nerve entrapment is defined as the prolonged compression on a peripheral nerve at any point along its course due to extrinsic or intrinsic mechanical forces(4). Symptoms frequently arise from a combination of nociceptive and neuropathic pain1. Initially, these cause paresthesias and nocturnal pain(5), and as they become chronic, numbness, burning sensations(6), electric-like pain with proximal radiation(7), and muscle weakness or atrophy in advanced cases(4). Occurrences of these mononeuropathies are increasingly common in clinical practice; however, it remains an underdiagnosed condition with low recurrence8. Additionally, the variability of etiologies and clinical manifestations, as well as the deficit in anatomical knowledge by clinicians, often pose a diagnostic challenge(9).
Sometimes conservative treatment proves to be ineffective, leading to the inclusion of therapies from specialties such as physical therapy to treat patients with chronic pain(10). Neuromodulation is a relatively modern technique defined by the International Neuromodulation Society as “the alteration of nerve activity through targeted delivery of a stimulus, such as electrical stimulation or chemical agents, to specific neurological sites in the body”. This somewhat outdated definition is updated by Albornoz M. and Maya J., who define this technique as “the use of advanced technologies to enhance or suppress nervous system activity in the management of diseases, including implantable or non-implantable devices” (11).
Among the current procedures described, focusing on their application on the Peripheral Nervous System (PNS), electrical stimulation can be applied transcutaneously (TENS) or percutaneously, either temporarily (PENS) or implanted (PNS) (12). PENS is defined by Fidalgo et al. (13) as “the electrical stimulation of a peripheral nerve using a needle as an electrode to reduce pain and restore neuromuscular and nervous system functions”.
As health care professionals, we must keep our knowledge up to date to provide the best possible health care to our patients. Therefore, with this work, we aim to obtain the information and evidence necessary to put our knowledge into practice in the future.
Objectives
Primary endpoint
A bibliographic review was conducted to assess the effectiveness of the percutaneous electrical stimulation technique in the management of neuropathic/chronic pain caused by the most frequent nerve entrapment syndromes in the foot and ankle.
Secondary objectives
Material and methods
To conduct this bibliographic review, a total of 29 articles and 1 book compiled and selected from February through March 2023 were evaluated through the databases of Pubmed, Dialnet, Science Direct, Scopus, and the Health Library of Universidad de Sevilla in Seville, Spain.
The keywords used were: “nerve entrapment”, “pain”, “neuromodulation”, “PENS”, “Foot” and “ankle”. All of them were combined using the Boolean operators “AND” and “OR”.
Inclusion criteria:
Exclusion criteria:
The broad time span was selected based on the scientific relevance of these studies, taking into account the number of citations, the relevance of the topic addressed, the protocols, and the method of application used, which may be feasible to apply in the field of podiatry, as well as the limitations present in current research.
Results
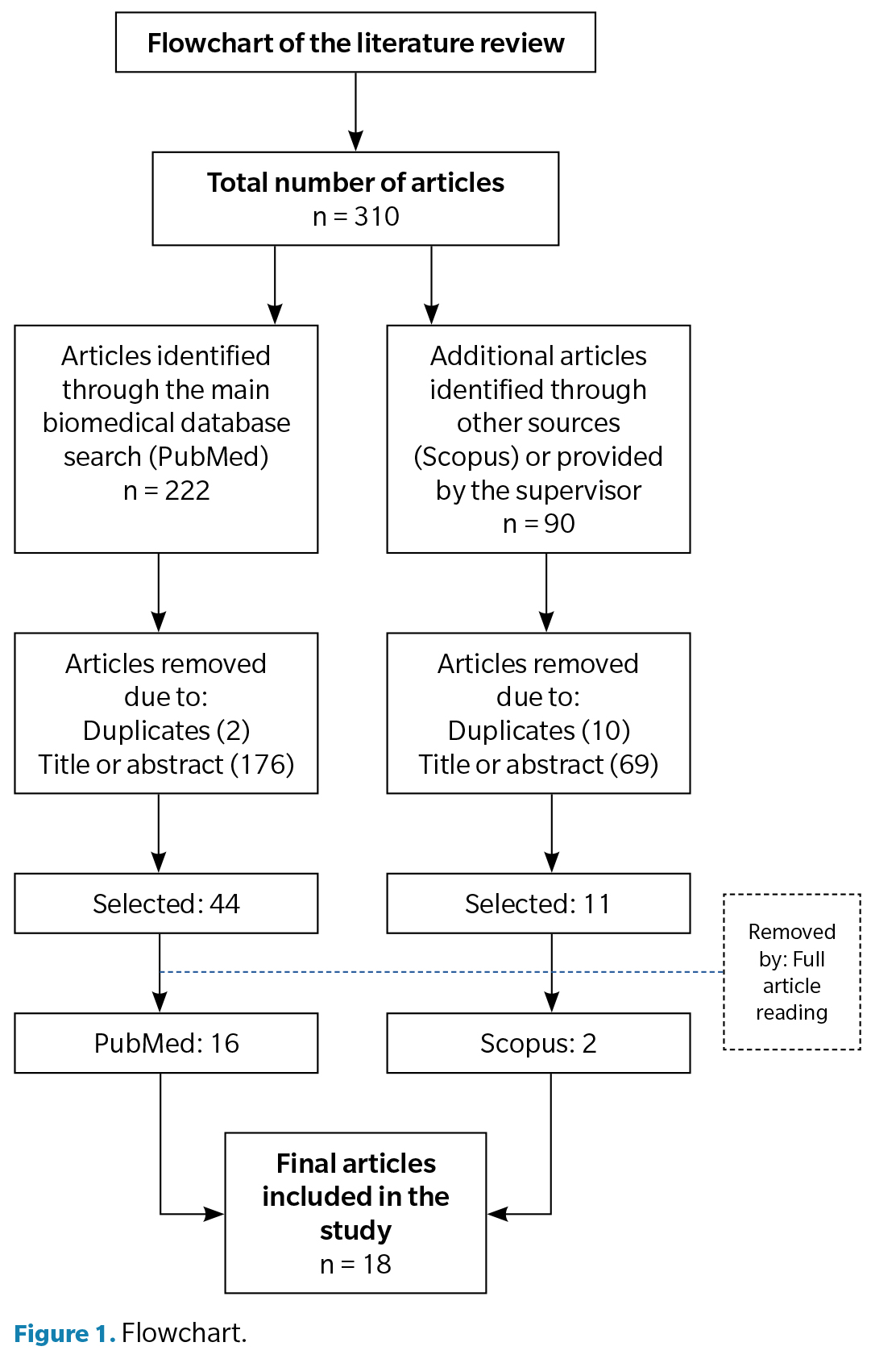
For the assessment of the results, a total of 18 articles were selected. To represent that the results were obtained in a more schematic way, a flowchart was developed (Figure 1).

Efficacy of PENS therapy for the management of pain
Musculoskeletal pain
Firstly, in their study, Plaza et al. (14) suggest that PENS therapy, when applied as a sole treatment, has a moderate effect on pain and related disability. Additionally, they found that combined with other interventions PENS therapy proved to be more effective, although this assumption seemed to be population-dependent. These results showed its effectiveness, although the evidence was scarce and evaluated in the short term.
Fidalgo et al.(13) reviewed its application in neuromusculoskeletal injuries and found that the technique significantly reduced pain and improved other aspects such as muscle strength and endurance, functionality, balance, disability, and medication intake.
Rodríguez et al.(15) evaluated the effects of TENS and PENS therapy on endogenous pain mechanisms in patients with acute or chronic musculoskeletal pain. They concluded that both techniques produce an immediate effect from mild to moderate in local mechanical hyperalgesia, although it could not be determined if these effects persisted over time.
Chronic low back pain
Weiner et al. (16) found that PENS therapy administered twice a week for a month and a half reduced pain and improved physical function for six months. Additionally, therapy was more effective when combined with aerobic exercises and physical therapy for six weeks.
Ghoname et al. (17) demonstrated that PENS was more effective than TENS and exercise in the short term, with an immediate pain reduction of 82 %, vs. 26 % and 4 % for TENS and exercise, respectively. Additionally, PENS reduced drug use by 50 %.
Years later, Yokoyama et al.(18) determined that PENS was more effective after 4 sessions, and when applied twice a week for 8 weeks, pain relief persisted for the following 2 months.
Neuropathic pain
Neuromodulation has become a good treatment option when the disorder is resistant to other therapies and phenomena of sensitization and hyperexcitability have established(19).
Raphael et al.(20) reduced chronic neuropathic pain and superficial hyperalgesia in 31 patients from 7.5 down to 0.5 on the numerical pain scale (NRS), demonstrating the short-term analgesic efficacy of stimulation.
Years later, Rossi et al.(19) evaluated a new PENS device in 76 patients with chronic peripheral neuropathic pain resistant to medication. Their study demonstrated effectiveness and safety in the short, medium, and long term, providing months of pain relief.
Postoperative pain
Ilfeld et al. (21) applied electrical currents through the implantation of a probe over the sciatic nerve, femoral nerve, and brachial plexus in foot, ankle, anterior cruciate ligament, and rotator cuff surgery. Stimulation was performed for 14 days after surgery at 100 Hz and 2 cm from the epineurium. As a result, pain scores and opioid consumption were reduced, at least, within the first week after surgery.
Despite the benefits in analgesia, probe implantation may carry risks such as infection, rupture, cable detachment, or nerve damage. In addition to requiring surgery and regular check-ups, just one qualified surgeon is all it takes to perform it(22). A less invasive therapy based on its efficacy in musculoskeletal and neuropathic pain, such as PENS, could be a valuable option for acute postoperative pain. This could reduce risks and improve patient satisfaction, for example, in surgeries such as tarsal tunnel approach with persistent postoperative pain.
PENS parameters for pain
The choice of parameters is controversial due to the lack of a universally accepted protocol. Frequency is considered the most relevant parameter according to most authors, despite the diversity of literature on the subject (10,13). As Beltrá et al. (22) explain, synaptic plasticity is “the ability of synapses to strengthen or weaken over time in response to increases or decreases in their activity.” There are two mechanisms of synaptic plasticity with different objectives: depressing the nociceptive pathway (LTD or long-term depression) or enhancing the non-nociceptive pathway (LTP or long-term potentiation).
Klein et al. (23) sought to evaluate long-term neuronal modulation in pain perception following LTD and LTP protocols on the nociceptive pain pathway, i.e., on C fibers. They applied a continuous pulse train, 1 Hz for 16 minutes and 40 seconds in 2 different intensities: 10 and 20 times the detection threshold of each individual. The application of high-intensity protocol on the nociceptive pathway elicited painful stimuli, increased neuronal responses in the dorsal horn of the spinal cord, and generated vasodilation, suggesting it could contribute to neurogenic hyperalgesia and chronic pain. On the other hand, the low-intensity protocol reduced pain perception, providing analgesia for over an hour by inducing depression of nociceptive fibers, which could be beneficial in patients with chronic pain.
Based on Melzack and Wall’s “gate control” theory proposed in 1965, Sdrulla et al. (24) investigated how long-term synaptic potentiation (LTP) of Aβ fibers (non-nociceptive pathway) affects responses of the nociceptive pathway by inducing prolonged inhibition of evoked excitatory postsynaptic current (eEPSC) by afferent arrival of high-threshold fibers in substantia gelatinosa neurons.
They used different stimulation frequencies and found that higher frequencies (50 and 1000 Hz) caused greater inhibition of eEPSCs in both healthy and nerve-damaged animals.
This protocol would be interesting to use in patients with chronic or neuropathic pain whose ability to activate the descending pain inhibitory pathway has been diminished, as this protocol could be used to induce that endogenous analgesia by enhancing AB fibers and the inhibition of nociceptive transmission from C fibers in the dorsal horn of the spinal cord.
Studies by Klein et al. (23) and Sdrulla et al. (24) provided the basis for the clinical trial conducted by Beltrá et al. (22), in which they aimed to evaluate the sensory and motor effects of both protocols in individuals without previous disease to determine which one was superior regarding the reduction of pain perception. They assessed the effects of these immediately and 24 hours after stimulating the median nerve. The authors developed a new LTP protocol based on previous findings of Sdrulla et al. (24). They found that by using 100 Hz pulses in 5 bursts of 5 seconds separated by 55-second rests they could induce synaptic depression on the nociceptive pathway without having to increase frequency. In turn, they created an LTD protocol consisting of the stimulation at 2 Hz for 16 minutes. As a result, the LTP protocol led to distal hypoalgesia without affecting motor performance or producing any painful perception. On the other hand, the LTD protocol did not cause hypoalgesia, reduced strength, and individuals did perceive discomfort.
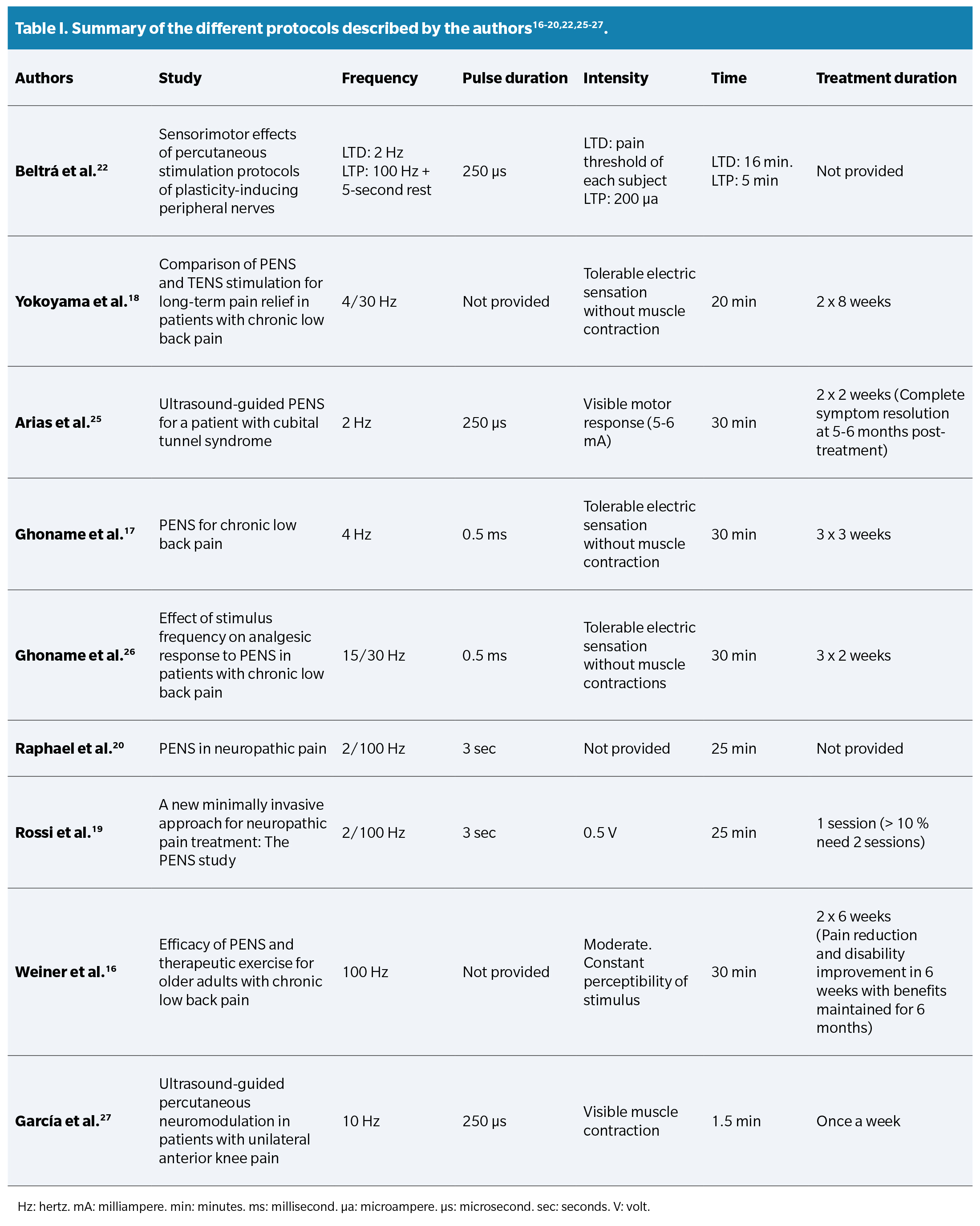
When investigating the opinion of other authors about the different application parameters, diverse perspectives and viewpoints were found. These protocols are summarized in a Table 1.
Regarding the application time, both Beltrá et al. (22), Yokoyama et al. (18), Arias et al. (25), and Fidalgo et al.13 agree that longer applications (>15 minutes) provide better clinical results. Authors such as Ghoname et al.26, Fidalgo et al. (13), Plaza et al. (14), Rossi et al. (19), Raphael et al. (20), and Yokoyama et al.18 agree that the combination of high and low frequencies improves therapeutic effectiveness by activating opioid receptors (μ, δ, and κ) and consequently different types of opioids related to “gate control” (dynorphins, enkephalins, or endorphins). However, the difference lies in that the LTP protocol by Beltrá et al. (22 includes short breaks between impulses to avoid continuous depression at the medullary level, while other protocols do not.
In the future, a comparative study with a larger sample size would be valuable to increase scientific evidence and facilitate the clinical application of these protocols.
Efficacy of PENS technique to reduce pain caused by nerve entrapment syndromes
Ferreira et al. (28) addressed a patient with chronic lumbosacral radiculopathy that did not respond to previous treatments, including spinal cord stimulation, who underwent percutaneous placement of a superficial peroneal nerve stimulator. In just two weeks, the patient experienced a decrease in pain, from 8/10 down to 1/10 on the NRS scale, along with improvements in mobility. Analgesia persisted when reassessed after three months. These results suggest that this technique may be an effective alternative for patients with nerve compression who have failed to respond to other therapeutic options.
In another case, Langford et al. (29) presented the case of a 57-year-old man with chronic meralgia paresthetica. After pharmacological treatment caused side effects, he underwent surgically implanted peripheral stimulation, which reduced his pain (from 8/10 down to 0/10 on the NRS scale). Despite device removal after 2 months, pain relief persisted for 1 year.
In 2020, García et al. (27) conducted a randomized clinical trial to investigate the short-term effects of PENS technique by applying it to the femoral nerve in patients with nonspecific anterior knee pain, considering it could be due to nerve entrapment, among other hypotheses. The results indicated improvements in the patients’ pain, functionality, and range of motion for one week after a single stimulation. The authors suggest applying this therapy weekly, although they do not specify the total duration of the treatment.
That same year, Fernández et al. (30) presented a case of a 48-year-old patient with neuropathy caused by cubital tunnel syndrome. Despite spending 1 year with symptoms, and a previous unsuccessful attempt with conservative treatments for six months, the patient underwent 3 PENS sessions once a week. In each session, a frequency of 2 Hz was applied for 30 minutes. Improvement was observed after the third session, leading to the addition of active sliding exercises for 2-3 weeks. One month after the last session, the patient experienced improvement that lasted for a year without the need for any additional interventions.
In 2019, Arias et al. (25) presented the case of a 43-year-old patient initially diagnosed with lateral epicondylalgia. After experiencing improvement for two years, he relapses after hitting himself while doing physical activity. Despite conservative treatments, physical therapy, and exercises, the pain persisted, with electrical qualities and irradiation to the forearm. Current clinical symptoms suggested the presence of possible radial tunnel syndrome. After intervention with PENS on the trunk of the radial nerve and the posterior interosseous nerve, in addition to a 4-week exercise program, the patient experienced significant improvement in pain and function, achieving complete symptom resolution in the subsequent 5-6 months.
Discussion
The PENS technique appears promising to treat nerve entrapments, although current evidence is limited, mainly based on case reports. Additionally, the lack of specific research in the foot and ankle represents a gap in specialty knowledge.
From the beginning of our research, we sought to expand our knowledge by exchanging experiences with physical therapy and podiatry professionals who use the PENS technique. Initially, we consulted with a physical therapist experienced in nerve stimulation to acquire the necessary knowledge, given the lack of depth with which this topic is addressed in Podiatry degree programs. However, upon further review of the currently existing literature, we noticed the lack of studies supporting the efficacy of PENS in treating pain caused by nerve entrapments in the foot and ankle. As a result, we contacted these specialists with the intention of witnessing firsthand the good results they all reported regarding its application in different cases of nerve entrapments such as Morton’s neuroma, Hausser’s neuroma, or Tarsal Tunnel Syndrome, among others.
We have been able to confirm the high level of patient satisfaction regarding pain reduction and improvement in functionality, both in the case reports provide and those we have witnessed. Although the results obtained based on each professional’s experience are positive, we should mention that each of them uses different action protocols regarding parameters/dosage (frequency and time), needle placement, or type of device, among others.
Finally, and for this reason, we consider it appropriate to conduct a future study with the appropriate methodological design to evaluate the effectiveness of the PENS technique for pain caused by the most frequent nerve entrapments in the foot and ankle, with the intention of documenting and demonstrating the good results we have been able to observe in the routine clinical practice.
Conclusions
The technique has demostrated short-term effectiveness in reducing musculoskeletal, neuropathic, or postoperative pain, as well as medication intake. Established authors use different action protocols. However, most alternate high and low frequencies within a 20-30 minute interval. There are difficulties in demonstrating the effectiveness of the PENS technique in nerve entrapment syndromes in the foot and ankle, although evidence exists for those occurring in other parts of the body.
Conflict of interest
The authors declare that they have no conflicts of interest.
Funding
None.
Authors’ contributions
Conception and study design: LRF.
Data collection: LRF.
Result analysis and interpretation: LRF.
Creation, drafting, and preparation of the initial draft of the paper: LRF, MMF.
Review and final acceptance: LRF, MMF, RMD.
References