10.20986/revesppod.2025.1701/2024
REVISIÓN
Effectiveness of botulim toxin in severe hiperhidrosis compared to traditional topical treatments. A narrative literature review
Eficacia de la toxina botulínica en la hiperhidrosis severa frente a tratamientos tópicos tradicionales. Revisión bibliográfica narrativa
Javier Vázquez González1
Laura Regife Fernández1
José María Juárez Jiménez1
1Departamento de Podología. Facultad de Enfermería, Fisioterapia y Podología. Universidad de Sevilla, España
Abstract
Severe hyperhidrosis is excessive sweating, not related to physical activity or increased temperature, which affects a higher percentage of the population than is commonly known. Its high prevalence in early adulthood impacts the quality of life and the social and occupational capacity of those who suffer from it. Additionally, the wide variety of existing treatments and the poor, inconsistent, or absent response of many of them complicates the choice and favors secondary complications from an unresolved process. Thus, the main objective is to analyze the use of Botulinum Toxin in hyperhidrosis in general, and in plantar localization in particular, through a narrative literature review.
A search was conducted in various scientific databases during the months of February and June 2023. After applying inclusion and exclusion criteria, a total of 32 articles were selected and analyzed. Of these, 13 met the search criteria and were included in the discussion.
The conclusions indicate that Botulinum Toxin is a highly effective technique, very satisfactory, and has a low complication rate for the treatment of hyperhidrosis. However, a greater number of studies are needed to determine its efficacy, optimal dosage, and duration of treatment in plantar areas.
Keywords: Antiperspirants, aluminum chloride, botulinum toxin, cholinergic antagonists, hyperhidrosis, iontophoresis, axilla, neuromuscular blockers
Resumen
La hiperhidrosis severa es una sudoración excesiva, no relacionada con la actividad física o el aumento de la temperatura, que afecta a la población en mayor porcentaje de lo que conocemos. La alta prevalencia en etapas tempranas de la adultez condiciona la calidad de vida y la capacidad social y laboral de las personas que la padecen. Además, la amplia variedad de tratamientos existentes y la escasa, nula o inconstante respuesta de muchos de ellos dificulta la elección y favorece las complicaciones secundarias a un proceso no resuelto. De esta manera, el objetivo principal es analizar el uso de la toxina botulínica en la hiperhidrosis en general y en la localización plantar en particular, mediante una revisión bibliográfica narrativa.
Se ha realizado una búsqueda en diversas bases de datos científicas durante los meses de febrero y junio de 2023. Tras aplicar criterios de inclusión y exclusión, se seleccionaron y analizaron un total de 32 artículos. De estos, 13 cumplieron con los criterios de búsqueda y se incluyeron en la discusión.
Como conclusiones, se obtiene que la toxina botulínica es una técnica altamente eficaz, muy satisfactoria y posee baja tasa de complicaciones para el tratamiento de la hiperhidrosis. Sin embargo, es necesario mayor número de estudios para determinar su eficacia, dosis óptima y duración del tratamiento en zonas plantares.
Palabras clave: Antitranspirantes, cloruro de aluminio, toxina botulínica, antagonistas colinérgicos, hiperhidrosis, iontoforesis, axila, bloqueantes neuromusculares
Corresponding author
Laura Regife Fernández
lauraregife@gmail.com
Received: 21-06-2024
Accepted: 30-12-2024
Introduction
While sweating is a mechanism in our body that allows us to regulate body temperature, cope with stress, and assist in metabolism, an excess of it leads to various physical, psychological, and social alterations, as well as infections, rashes, and skin maceration, or difficulties in social (reflected by 75 %) or workplace environments (80 % of patients) (1).
Hyperhidrosis affects approximately 3 % of the U.S. population(2) or 1 % of the global population(3,4), although it is believed that this number may be much higher due to a large number of unreported or misdiagnosed cases(2). It is more commonly seen in adults, with no differences between genders, typically between 18 and 40 years old, with a minor proportion in children, adolescents, and the elderly—likely due to its regulation over time(1).
Hyperhidrosis can be divided into primary or secondary. When it is not associated with systemic pathologies, body temperature, or other external stimuli, it is referred to as primary hyperhidrosis. On the other hand, if the cause is drugs, malignant tumors, endocrine disorders, or other problems of the central nervous system, it is classified as secondary hyperhidrosis(1).
Between 35 % and 55 % of primary hyperhidrosis cases have a hereditary component(1,5), typically appearing between the ages of 14 and 25, whereas secondary hyperhidrosis generally manifests after the age of 25 and is not strongly linked to family episodes(1).
Hyperhidrosis that causes a severe deterioration in quality of life is considered severe and is reported by many patients (scoring 4) through tolerance scales ranging from 1 to 4, where 1 does not interfere with daily activities, and 4 is intolerable. This leads to the selective choice of activities compatible with the condition(1).
Although hyperhidrosis is not considered a severe disorder, it poses challenges in treatment and long-term efficacy. Botulinum toxin emerges as a novel and effective option to treat this condition. This relatively new therapeutic approach allows for an in-depth exploration of its mechanisms of action and efficacy, setting new standards compared to traditional treatments.
The application of botulinum toxin requires specialized technical knowledge and the use of advanced imaging and injection techniques. These technological advances have improved the understanding of sweating physiology and the toxin’s mechanisms of action, rapidly increasing its use and benefiting many patients. Due to its clinical relevance, therapeutic innovation, and associated technological advances, botulinum toxin has become a topic of great interest for both healthcare professionals and patients. It is crucial to delve into the clinical and therapeutic aspects of its application, highlighting its potential as an effective solution for such a common condition.
The main aim is to analyze the use of Botulinum Toxin in hyperhidrosis in general and specifically in plantar localization, through a narrative literature review. The secondary endpoints are to evaluate the effectiveness of other topical treatments in managing hyperhidrosis and to review how the localization of hyperhidrosis affects treatment selection.
Materials and methods
For this narrative literature review, a total of 32 articles were evaluated, selected between February and June 2023 using the PubMed, Dialnet, and Scopus databases.
The keywords used were: “antiperspirants,” “aluminum chloride,” “botulinum toxin,” “cholinergic antagonists,” “hyperhidrosis,” “iontophoresis,” “axilla,” and “neuromuscular blockers.” All were combined using the boolean operators “AND” and “OR.”
Inclusion criteria
- Bibliographic, systematic reviews and/or meta-analyses, randomized or non-randomized clinical trials, observational studies, book chapters, or case reports.
- Articles published from 2010 to the present.
- Studies focused on severe hyperhidrosis in the axillae, hands, or feet, examining the use of botulinum toxin and other topical, systemic, and invasive treatments.
Exclusion criteria
- Articles not related to the topic described in this work.
Results & Discussion
To assess the results, a total of 13 articles were selected. To present these findings more systematically, a flowchart was created (Figure 1).
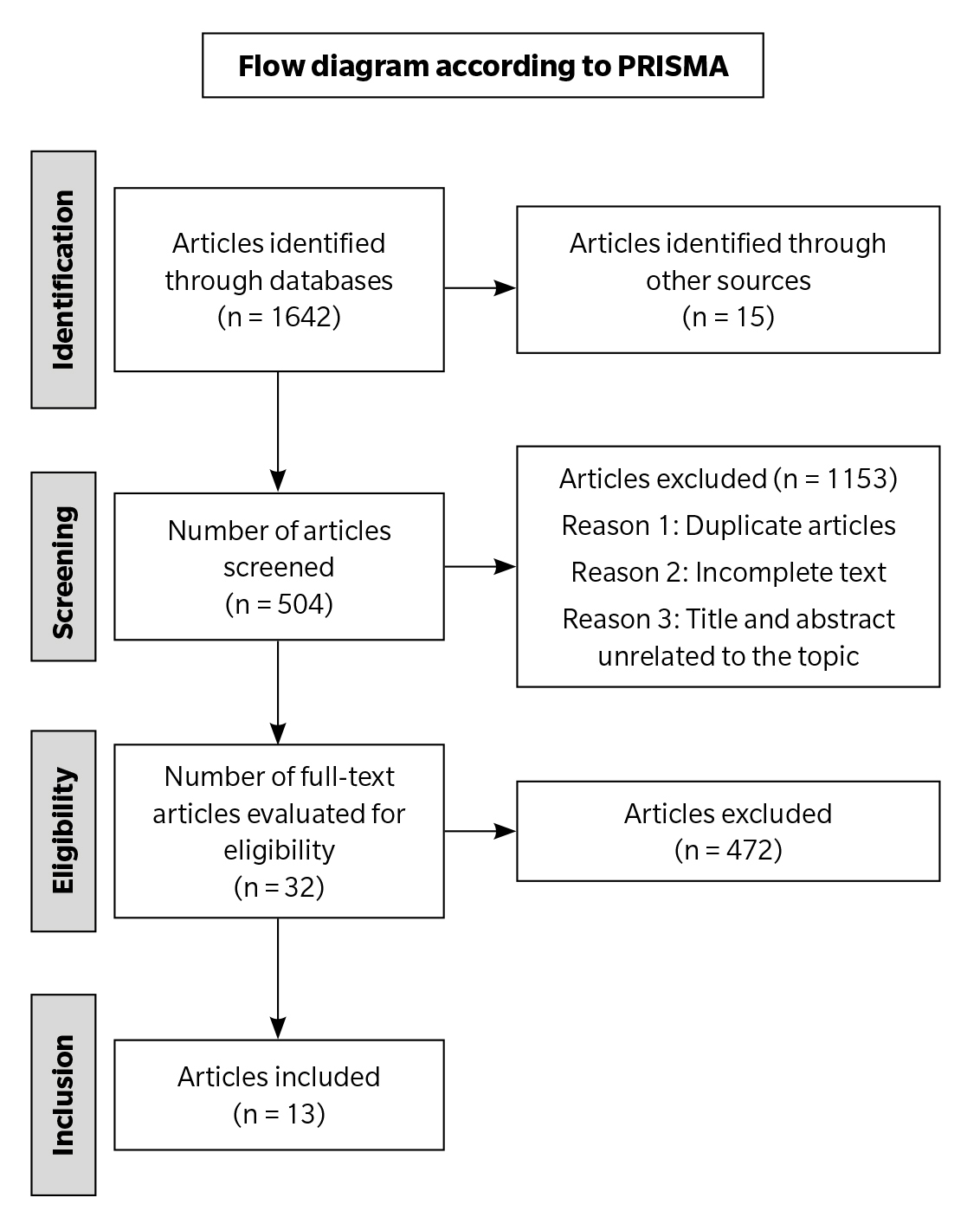
Figure 1. Flow diagram according to PRISMA.
Botulinum toxin and hyperhidrosis
Hyperhidrosis, characterized by excessive sweating in various body regions, causes physical, emotional, and social issues that require therapeutic intervention. Botulinum toxin type A, increasingly used, inhibits acetylcholine release in sympathetic nerves, impacting eccrine glands(6). This treatment offers fewer complications and greater efficacy than other methods. Its inhibitory effect lasts 3 days, after which nerve regeneration begins within 7 days, with full functionality restored in 3 to 6 months(7).
Four types of botulinum toxin type A are FDA-approved for therapeutic and cosmetic use. However, only OnabotulinumtoxinA is specifically approved for treating severe axillary hyperhidrosis(7). Each type differs in the associated proteins in its formulation. The FDA has also approved a type B botulinum toxin, derived from another bacterial strain, particularly useful for cases where immune resistance to type A has developed, potentially neutralizing its effects(6,7).
Type B toxin acts more rapidly than type A and shows higher affinity for autonomic nerve terminals rather than neuromuscular ones, making it an intriguing option. However, comparative evidence is needed to establish equivalent dosing between types A and B(6).
The application methodology for botulinum toxin in primary hyperhidrosis treatment has remained consistent. According to established protocols, intradermal injections are administered at the dermal-subcutaneous junction, in areas pre-marked using the starch-iodine test, with injection depth varying by location: 4.5 mm on the sole of the foot and 2 mm in the axillae. Overly deep injections may affect deeper nerve branches not involved in sweating. The administration uses a hypodermic needle angled between 30° and 45°, with the bevel facing the operator(7).
Awaida et al. (8) propose a modification to the injection pattern, suggesting fewer punctures reduce pain by administering the same dose in 7 injections instead of 20. While higher volumes per injection might be expected to cause more pain, patients report less discomfort, likely due to reduced anxiety associated with fewer punctures, while maintaining the efficacy of botulinum toxin.
The primary challenge in administering these injections is pain, especially in the hands, leading some patients to prefer less invasive therapies, reserving botulinum toxin injections for cases where other options have failed. Various authors propose techniques to mitigate pain, including nerve blocks, ice application, analgesic vibration, and topical cooling devices?. Vlahovic emphasizes the importance of local anesthesia with lidocaine, nerve blocks, and/or ice to minimize discomfort(10). Nawrocki et al. (7) suggest adding lidocaine to the botulinum toxin solution to reduce pain.
For hands, nerve blocks target the median, radial, and ulnar nerves, all originating from the brachial plexus, which provides motor and sensory innervation to the upper limb¹¹. For feet, blocks target the sural and posterior tibial nerves, which innervate the sole(7).
Botulinum toxin administration for hyperhidrosis can be associated with various complications. Obed et al. (12) note that injection pain is the primary issue reported by patients. It is essential to inform patients about possible adverse effects such as muscle weakness, which may persist for up to four months(7), as well as the minimal risk of anaphylactic reactions.
Botulinum toxin treatments should be avoided in pregnant or breastfeeding women, as it is classified as a category C drug during pregnancy, and in patients with hypersensitivity to its components or local infections at the treatment site(7,10). Its use is also not recommended in secondary hyperhidrosis, coagulation disorders, or in those who have undergone sweat gland removal surgery(8).
Patients with pre-existing amyotrophic lateral sclerosis, peripheral neuropathy, or neuromuscular junction disorders such as myasthenia gravis or Lambert-Eaton syndrome should be closely monitored, and the concomitant use of medications that may alter botulinum toxin metabolism should be avoided(8).
Regarding specific adverse effects, studies show that botulinum toxin type A rarely causes allergic reactions, skin necrosis, hemorrhages, or systemic muscle weakness, although it can cause pain and burning sensations during application, according to Awaida et al. (8) In another study by Wade et al.,¹³ no severe adverse reactions were reported, with injection site pain (axillae) being the most common complication, affecting up to 12% of patients, along with one case of compensatory facial hyperhidrosis.
In contrast, botulinum toxin type B appears to be associated with a higher frequency of side effects. In a study cited by Wade et al., (13) involving 20 patients, 83 adverse reactions were reported, including reduced hand grip strength (50 % of patients), muscle weakness (60 %), dry mouth (90 %), excessive hand dryness (60 %), and indigestion (60 %). Nawrocki et al.? mention that type B toxin shows a higher prevalence of side effects such as dry mouth, headaches, corneal irritation, general discomfort, and sensory and motor disturbances compared to type A.
In hyperhidrosis treatment with botulinum toxin, there is no standardized protocol for exact dilutions. According to Nawrocki et al. (7), OnabotulinumtoxinA is typically diluted between 1 and 10 mL, with 2 to 5 mL being most common. AbobotulinumtoxinA is generally diluted between 2.5 and 5 mL, though ranges can vary from 1.25 up to 10 mL.
Doses vary by treatment area. For axillae, 50U of OnabotulinumtoxinA are typically applied in 0.1 to 0.2 mL injections per site, adjusted to the area’s size. For palms, higher concentrations (75-100U) in smaller volumes (0.05-0.1 mL) are used, administered in up to 50 points due to pain. For plantar hyperhidrosis, 100 to 200U of OnabotulinumtoxinA per foot are required(7).
Regarding the comparison between botulinum toxin types A and B, An et al. (6) suggest a 1:30 ratio, meaning that for every 50U of type A toxin, 1,500U of type B would be required to achieve a similar inhibitory effect. This approach may remain effective up to 20 weeks after the initial treatment.
Botulinum toxin is preferred by hyperhidrosis patients due to its prolonged efficacy vs short-term conventional treatments. According to Vlahovic, symptoms improve by up to 75% over six months. Similarly, a study of 2 patients conducted by Tamura indicated symptom relief starting at 14 days and lasting up to six months(10).
Wade et al. (13) reported improvements of 57 % at 2-4 weeks and 67 % at 16 weeks post-treatment vs placebo. Obed et al. (13) highlighted significant reductions in sweating and improvements in quality of life up to 8 weeks after treatment, although long-term data is limited. In studies by Wade et al. (13), the mean duration of toxin effects in axillae ranged from 197 to 273 days, significantly longer than placebo effects (35 to 96 days).
In the case of Botulinum Toxin Type B, it has a faster onset of action than Type A but a shorter effective duration of 9 to 16 weeks(14).
Patient satisfaction and improved quality of life are key objectives in hyperhidrosis treatment. Comparative studies with placebos show moderate to substantial satisfaction in patients treated with botulinum toxin, contrasted with mild satisfaction in the placebo group(13). Moreover, botulinum toxin demonstrates significant advantages in addressing bromhidrosis, reducing the average odor level from 8 to 2 in treated axillae after three months, vs a maintained level of 8 in the placebo group. Similarly, sweat production significantly decreased in treated axillae, representing one-third of the initial level compared to the control axilla(41).
Botulinum toxin stands out as an effective option for reducing excessive sweating and improving patients’ quality of life, delivering quick and long-lasting results with a low incidence of mild and transient complications or side effects.
Most common topical treatments for severe hyperhidrosis
Botulinum toxin variants have been extensively compared in numerous studies regarding techniques, dosages, and efficacy relative to placebos, aiding in evaluating their effectiveness, duration, and complications. To better understand their curative capacity compared to other available treatments, this review focuses on the two most common topical therapies: aluminum salts and iontophoresis, which are first- and second-line therapies, despite the availability of oral, systemic, surgical, and physical options.
Aluminum salts are widely used due to their low cost and ease of application but are not ideal for treating moderate or severe hyperhidrosis, as they provide short-term relief and require regular application. They can cause irritation and skin dryness¹². These salts work by blocking sweat gland ducts through the interaction of aluminum metal salts with sweat mucopolysaccharides. They are applied as a lotion every 24–48 hours before bed, with visible effects in 1-2 weeks. Subsequently, application frequency is reduced to 1-2 times weekly for maintenance. Patients report high satisfaction in axillae (94 %) and soles (84 %), but less so for palms (60 %)(15).
Iontophoresis is a safe and effective treatment for hyperhidrosis, where hands or feet are submerged in water while an electrical current is applied, reducing sweating through ion exchange¹?. Session frequency varies from daily to weekly, with intensities of 0 to 30 mA and durations of 10 to 30 minutes¹³. However, the need for frequent treatment repetition can be frustrating for patients(16).
Wade et al. (13) reported a 43 % reduction in sweating after 28 days of treatment and 83 % after 3 months in different studies using botulinum toxin. Adverse reactions are minimal and can be avoided with proper patient education, although they may include vesiculation, erythema, discomfort, and dryness, which can be mitigated with moisturizers and intensity adjustments. Contraindications include pregnancy, intrauterine devices, hypoesthetic areas, pacemakers, metal implants, cardiac conditions, epilepsy, and skin lesions(10,4).
When comparing botulinum toxin with iontophoresis in patients with palmar hyperhidrosis, Wade et al. (13) found that botulinum toxin type A (100U per palm) showed greater improvement in sweat reduction: 57 % of patients improved with botulinum toxin versus 27 % with iontophoresis. Additionally, 80 % of patients reduced sweating with botulinum toxin compared to 47% with iontophoresis. Another study by the same author showed a 90.9 % improvement with botulinum toxin versus 35.7 % with iontophoresis in the first month, without observing adverse reactions in the 86 monitored patients.
More updated studies are required to evaluate the effectiveness of treatments, as technology continuously improves and may offer better therapeutic methods for hyperhidrosis.
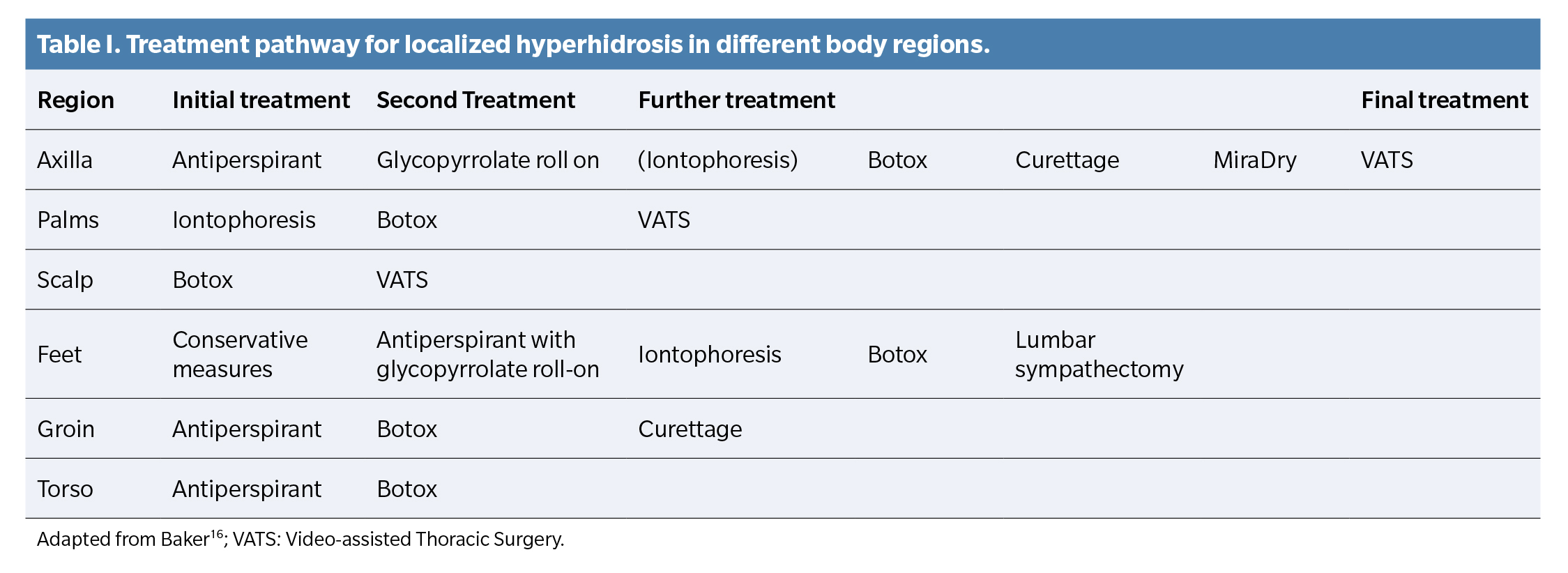
Review of hyperhidrosis treatment based on its location
There is no standardized algorithm for the treatment of hyperhidrosis based on location. Therapeutic decisions vary according to the author’s experience. Non-surgical treatments, such as topical antiperspirants, briefly relieve symptoms and often cause irritation¹². Iontophoresis is the next option when antiperspirants are ineffective and can be combined with them¹?. It is preferred for moderate to severe palmar-plantar hyperhidrosis(17).
Although clinical guidelines agree on the use of botulinum toxin after the failure of topical therapies, Obed et al. (12) affirm the high efficacy of the toxin compared to other first-line topical treatments. While their studies are insufficient to establish a new approach to managing hyperhidrosis, they confirm that the efficacy of other treatments is not superior to botulinum toxin.
Despite the high efficacy of botulinum toxin, its high cost limits its early use in treatment. (12) Based on acetylcholine, glycopyrrolate, as an oral treatment, helps control overall body sweating and does not produce compensatory sweating(12).
The International Hyperhidrosis Society does not recommend any type of sympathectomy for plantar hyperhidrosis due to the likelihood of side effects such as compensatory hyperhidrosis, heat intolerance, and the irreversibility of the surgical process(10).
Nawrocki et al. (15) prefer the use of topical antiperspirants as the first-line therapy for palms, axillae, and soles, while Baker¹? opts for iontophoresis, antiperspirants, and conservative measures, respectively.
As a second therapeutic option, Nawrocki et al. (15) recommend iontophoresis for palms and soles and botulinum toxin for axillae. Baker(16), however, differs in opinion, applying botulinum toxin for palms, antiperspirants for soles, and glycopyrrolate for axillae.
Each author adopts a therapeutic approach based on their experience and perspective, without following a uniform protocol, as can be observed in Figure 2 and Table 1
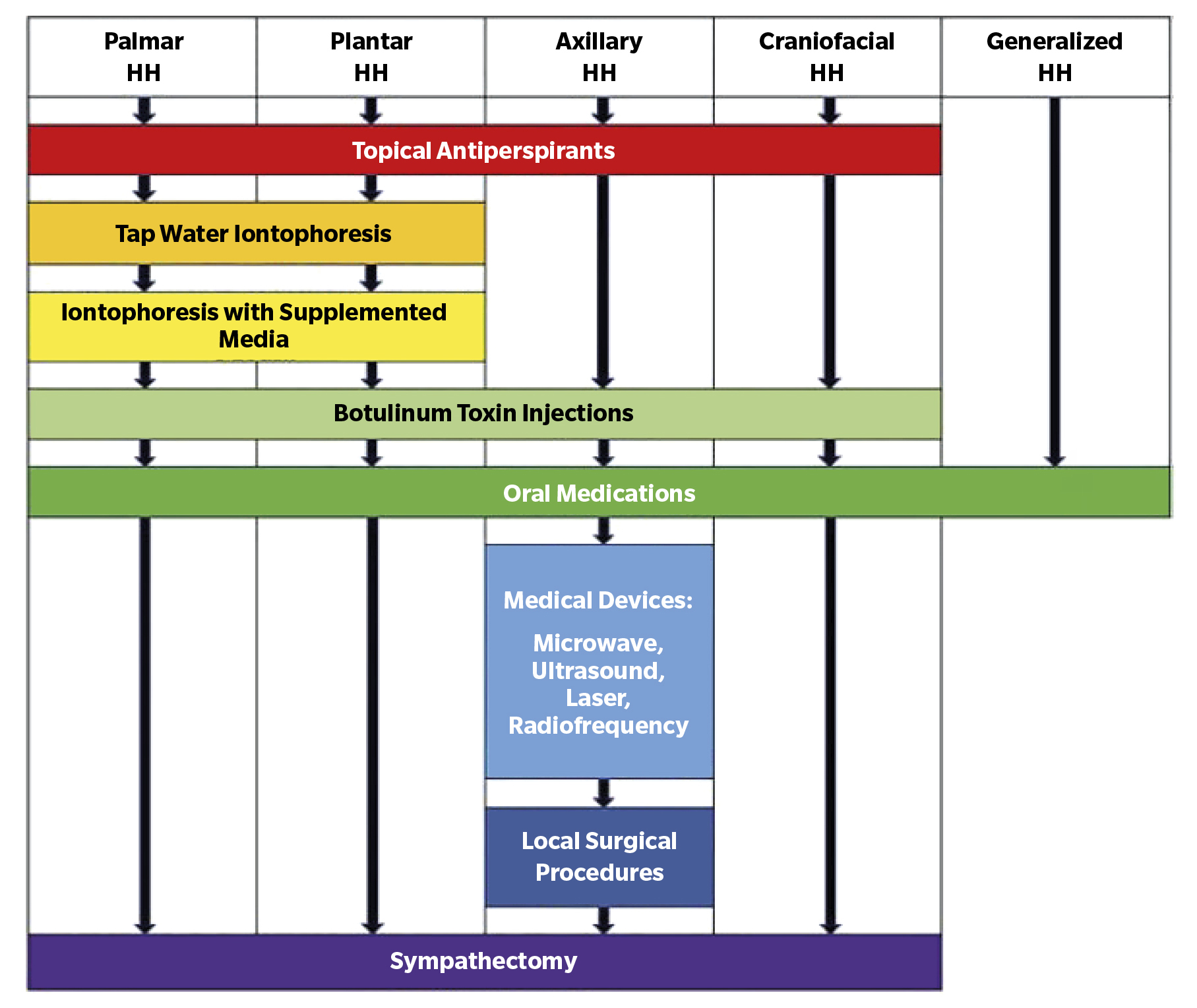
Figure 2. Treatment approach for hyperhidrosis patients. Adapted from Nawrocki & Cha(15).
The present review identified several limitations. No high-evidence studies were found that directly compare the outcomes of different techniques, leaving significant room for more detailed research with larger populations. Comparison across different treatments was complicated due to methodological diversity among authors. Additionally, proposed modifications to existing therapies by some authors lack verification by other researchers, limiting their value in establishing a reliable therapeutic guide. Therefore, more studies are necessary to improve the available evidence in this field.
As final conclusions, botulinum toxin infiltration proves highly effective in treating palmar and plantar hyperhidrosis, with a low incidence of complications and high patient satisfaction. The effects are usually temporary. Although recommended for plantar hyperhidrosis, more studies are needed to determine the optimal dosage and specific duration of each formulation. Moreover, iontophoresis and the use of aluminum salts generally offer non-invasive and accessible alternatives for treating hyperhidrosis, making them viable options before resorting to more invasive treatments or drugs. While they are easy to use and can be combined with other treatments to increase efficacy over time, their main limitations include the need for repeated treatments, similar to other therapies, and their lower efficacy vs other available options. Finally, establishing an exact treatment selection pattern for each hyperhidrosis location is complex due to the disparity of criteria among authors. However, most agree on the following recommendations: Axillae: Aluminum salts are suggested as the first option, followed by botulinum toxin. Palms: Iontophoresis is the first choice, followed by botulinum toxin. Soles: Although botulinum toxin is effective, the use of aluminum salts is recommended first, followed by iontophoresis.
Authors’ contributions
Study conception and design: JGV.
Data collection: JVG, LRF.
Data analysis and interpretation: JVG, LRF.
Creation, drafting, and drafting of the initial manuscript: JVG, JMJJ.
Final review: LRF, JMJJ.
Conflicts of interest
None declared.
Funding
None declared.
References
- Lenefsky M, Rice ZP. Hyperhidrosis and its impact on those living with it. Am J Manag Care. 2018;24(23):S491-S495.
- Hoorens I, Ongenae K. Primary focal hyperhidrosis: current treatment options and a step-by-step approach. J Eur Acad Dermatol Venereol. 2012;26(1):1-8. DOI: 10.1111/j.1468-3083.2011.04173.x
- Callejas MA, Grimalt R, Cladellas E. Actualización en hiperhidrosis. Actas Dermosifiliogr. 2010;101(2):110-8. DOI: 10.1016/j.ad.2009.09.004
- del Boz J. Systemic Treatment of Hyperhidrosis. Actas Dermosifiliogr. 2015;106(4):256-62. DOI: 10.1016/j.ad.2014.11.012
- Semkova K, Gergovska M, Kazandjieva J, Tsankov N. Hyperhidrosis, bromhidrosis, and chromhidrosis: Fold (intertriginous) dermatoses. Clin Dermatol. 2015;33(4):483-91. DOI: 10.1016/j.clindermatol.2015.04.013
- An JS, Won CH, Han JS, Park HS, Seo KK. Comparison of onabotulinumtoxinA and rimabotulinumtoxinB for the treatment of axillary hyperhidrosis. Dermatol Surg. 2015;41(8):960-7. DOI: 10.1097/DSS.0000000000000429
- Nawrocki S, Cha J. Botulinum toxin: Pharmacology and injectable administration for the treatment of primary hyperhidrosis. J Am Acad Dermatol. 2020;82(4):969-79. DOI: 10.1016/j.jaad.2019.11.042
- Awaida CJ, Rayess YA, Jabbour SF, Abouzeid SM, Nasr MW. Reduction of Injection Site Pain in the Treatment of Axillary Hyperhidrosis With Botulinum Toxin: A Randomized, Side-by-Side, Comparative Study of Two Injection Patterns. Dermatol Surg. 2021;47(1):154-7. DOI: 10.1097/DSS.0000000000002193
- Weinberg T, Solish N, Murray C. Botulinum neurotoxin treatment of palmar and plantar hyperhidrosis. Dermatol Clin. 2014;32(4):505-15. DOI: 10.1016/j.det.2014.06.012
- Vlahovic TC. Plantar Hyperhidrosis: An Overview. Clin Podiatr Med Surg. 2016;33(3):441-51. DOI: 10.1016/j.cpm.2016.02.010
- de Quintana-Sancho A, Conde Calvo MT. Tratamiento de la hiperhidrosis palmar con toxina botulínica mediante bloqueo de los nervios periféricos al nivel de la muñeca. Actas Dermosifiliogr. 2017;108(10):894-5. DOI: 10.1016/j.ad.2017.05.013
- Obed D, Salim M, Bingoel AS, Hofmann TR, Vogt PM, Krezdorn N. Botulinum Toxin Versus Placebo: A Meta-Analysis of Treatment and Quality-of-life Outcomes for Hyperhidrosis. Aesthetic Plast Surg. 2021;45(4):1783-91. DOI: 10.1007/s00266-021-02140-7
- Wade R, Rice S, Llewellyn A, Moloney E, Jones-Diette J, Stoniute J, et al. Interventions for hyperhidrosis in secondary care: a systematic review and valueof-information analysis. Health Technol Assess. 2017;21(80):1-280. DOI: 10.3310/hta21800
- Wu CJ, Chang CK, Wang CY, Liao YS, Chen SG. Efficacy and Safety of Botulinum Toxin A in Axillary Bromhidrosis and Associated Histological Changes in Sweat Glands: A Prospective Randomized Double-Blind Side-bySide Comparison Clinical Study. Dermatol Surg. 2019;45(12):1605-9. DOI: 10.1097/DSS.0000000000001906
- Nawrocki S, Cha J. The etiology, diagnosis, and management of hyperhidrosis: A comprehensive review: Therapeutic options. J Am Acad Dermatol. 2020;81(3):669-80. DOI: 10.1016/j.jaad.2018.11.066
- Baker D. Hyperhidrosis. Surgery (Oxford). 2022;40(1):70-5. DOI: 10.1016/j.mpsur.2021.11.001
- Delgado SG, Fanjul EG. Decálogo de iontoforesis para el tratamiento de la Hiperhidrosis. Enfermería Dermatológica. 2017;11(31):22-5.